Abstract

Introduction: Treatments for lymphoma have improved significantly over the last three decades, particularly since the advent of rituximab for non-Hodgkin lymphoma. Improved survival rates have led to an increasing number of lymphoma survivors. Many of the treatments for lymphoma as well as lymphoma itself are known to affect the immune system; yet, despite the range of immune perturbations lymphoma survivors may have experienced, very little is known about their mid- and long-term immune health. Therefore, we investigated whether survivors of diffuse large B-cell lymphoma (DLBCL) have altered risk of developing autoimmune and infectious diseases compared to other cancer survivors.
Methods: All 21,690 Californians aged ≥18 years when newly diagnosed with DLBCL during 1991-2012, surviving a minimum of 1 year from diagnosis and without evidence of HIV/AIDS were identified in the California Cancer Registry and followed in California hospital, emergency department and ambulatory surgery discharge databases through 2014. We categorized discharge diagnoses to classify autoimmune and infectious diseases. To avoid possible relationships to DLBCL pathogenesis, we excluded conditions identified prior to or within 1 year of cancer diagnosis from our incidence analyses. We calculated incidence rate ratios (IRRs) and associated p-values comparing the incidence of autoimmune and infectious diseases among female and male DLBCL survivors to that among breast and prostate cancer survivors >1-10 years after cancer diagnosis using multivariable Poisson regression models adjusting for age, race/ethnicity and year of cancer diagnosis. We estimated the cumulative incidence of developing new autoimmune and infectious disease conditions >1-10 years after diagnosis, accounting for death as a competing risk.
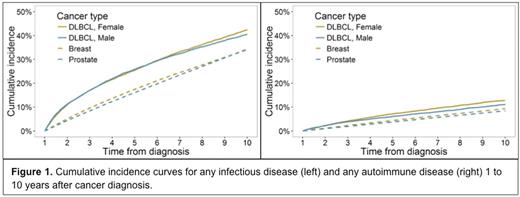
Results: Most DLBCL patients had at least one hospitalization prior to or within 1 year of diagnosis (67.9%) and >1-10 years of diagnosis (78.6%). Median follow-up time was somewhat longer for female breast (8.2 years) and male prostate (8.3 years) cancer survivors than for DLBCL survivors (female, 6.3 years; male, 5.9 years). In DLBCL survivors, we identified a higher cumulative incidence of any autoimmune or any infectious disease diagnosis than in breast or prostate cancer survivors (Figure 1). After adjustment for age, race/ethnicity and year of diagnosis, we found DLBCL survivors to have a more than two-fold higher incidence of fungal pneumonia (IRR 6.2 versus breast; IRR 8.2 versus prostate), viral pneumonia (IRR 3.4 versus breast; IRR 4.5 versus prostate), bacteremia (IRR 2.6 versus breast; IRR 3.1 versus prostate), and CNS infections (IRR 2.6 versus breast; IRR 3.2 versus prostate) (all p<0.0001). Compared to prostate cancer survivors, male DLBCL survivors had higher incidence of liver abscesses (IRR 3.0), empyema (IRR 2.4), bacterial pneumonias (IRR 2.1), and infections of implanted devices (IRR 2.1). For autoimmune diagnoses, we found DLBCL survivors to have a higher incidence of autoimmune hemolytic anemia (IRR 10.1 versus breast; IRR 8.7 versus prostate), immune thrombocytopenia (IRR 3.1 versus breast; IRR 4.7 versus prostate), and diffuse connective tissue diseases (IRR 2.1 versus breast; IRR 2.5 versus prostate) (all p<0.0001). In comparing male DLBCL survivors to prostate cancer survivors, we additionally found an increase in acquired coagulation factor deficiencies (IRR 2.1), caused by autoimmunity against clotting factors. Finally, we found that DLBCL survivors had a markedly increased incidence of being diagnosed with impaired humoral immunity (IRR 16.0 versus breast; IRR 14.0 versus prostate), perhaps a reflection of persistent hypogammaglobulinemia.
Conclusion: These findings in a large, population-based study suggest that compared to survivors of other common cancers, DLBCL survivors experience excess immune-related conditions, some expected (such as autoimmune hemolytic anemia and thrombocytopenia) and others unexpected (such as fungal and viral pneumonias and diffuse connective tissue diseases). This information suggests possible lasting effects of lymphoma and its treatments on the immune system and motivates further examination of immune function in DLBCL survivors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal